

When you stop to think about it, keeping your battery alive is a lot like
caring for a goldfish. You can ignore it in your haste and it won't make a
sound, but if you forget about it all together, you may be sad to find it's
dead. And when your battery is dead, the big difference is that your plans get
flushed down the toilet, not the fish's.
Because the battery is crucial to the starter motor (and kickstarters on streetbikes are about as common these days as acetylene headlights), and it's voltage is also often essential to the alternator's ability to generate sufficient juice to make sparks, starting a bikeeven bump startingwith a dead battery may be impossible. So, some practical knowledge about the care and feeding of that pet battery can make it either a motorcyclist's best friendor the option of the shoeleather express. Hitchiking and leaving your precious bike where it last ran could make battery ignorance anything from a big bother to a total terror in many parts of urban America.
You can make a simple wet-cell storage battery with only two lead plates. Submerge them in an electrolyte solution (64% water and 36% sulfuric acid by weight is standard), apply direct current and watch as the positive lead plate develops a brown coating of lead peroxide and the negative plate becomes sponge lead. Remove the voltage source and put a voltmeter across the plates and you'll find approximately 2.1 volts, regardless of the size. The larger the plates, the longer the battery can supply this voltage. Combining three such cells in series can create a 6-volt battery, and six cells can make a 12-volt battery (actually 6.3 or 12.6 volts, respectively).
When we ask the battery to produce current flow by putting a load across its terminals, the plates and acid solution undergo another chemical transformation, causing both lead plates to change into lead sulfate, consuming the acid and producing water as a by-product.
Gradually the electrolyte becomes increasingly watery and the plates more sulfated until the battery either dies or we reverse the process by returning current to the battery to restore it. The basic chemistry hasn't changed for a hundred years.
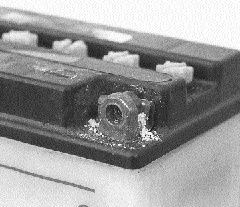
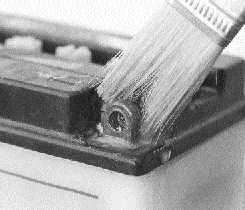
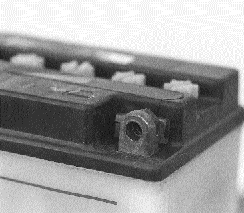
Crusty corrosion on this terminal is simply brushed with a solution of baking soda and water. The foaming solution dissolves the mess, then a blast of compressed air leaves everything spotless.
A modern motorcycle battery is a marvel of compact packaging. Since even a smooth-running motorcycle subjects the innards of a battery to much greater vibration than a car, the motorcycle battery's case will be a tighter fit to prevent the lead plates from rattling to pieces. But it's still generally true that a motorycle that vibrates a lot will have a shorter battery life, because the plates themselves are more fragile than you might expect. They are constructed of highly active but very soft lead pastes applied to waffled supporting grids, which provide greater surface area for the chemical reaction than simple flat plates. The paste is porous to allow full penetration of the electrolyte, and the pastes on both the negative and positive plates begin as the same substance; a mixture of lead oxide, dilute sulfuric acid, water and special binders like plastic fiber that produce a material about the consistency of firm mud. Since the negative plates tend to contract in service, special expanders are added to their mix so they can't shrink to become inpenetrable and chemically inactive. Just like our simple cell, the plates are then submerged in electrolyte and given a electrical charge that forms them into positive and negative.
To make the battery more rugged, the plate grids must be made of a harder lead alloy, usually lead-antimony. Anywhere from .5%12% antimony is typical, with higher antimony mixtures making for tougher plates but shortening the sitting life of the charged battery. The big drawback to lead-antimony is that gradual corrosion of the positive grid releases the antimony, which may then form tiny hairlike bridges between the plates. These bridges are actually short circuits that gradually increase the current necessary to recharge the battery, causing increased water loss. Therefore, an older battery needs its water level checked more frequently, and a new battery that needs constant filling may have a voltage regulator problem that is overcharging the battery.
Sealed, maintenance-free batteries use calcium instead of antimony to strengthen the plate grids, since calcium does not produce internal shorts. By giving the battery box a slightly larger volume to hold an extra reserve of electrolyte, incorporating sulfation-retardants and gas-recombinant technology (GRT: a special glass mat surrounding the plates which helps the hydrogen and oxygen recombine rather than escape from the battery as a gas), the manufacturer can eliminate the filler caps. However, all these batteries are not truly sealed, but may incorporate almost invisible safety vents around the perimeter of their tops. As advertised, the maintenance-free battery does have a natural resistance to overcharging and water loss. But unfortunately, they are also not as resilient when deeply discharged (considered to be the loss of 80% capacity) as are their lead-antimony brothers, and may be killed by such things as leaving an electrical load on for a long time (when a 12 volt battery might drop to just 2 volts), while a lead-antimony battery might still be saved. Some of the new high-tech "smart" battery chargers (about $60) claim to be able to restore even a deeply-discharged maintenance-free battery by increasing the initial charging voltage to as high as 20 volts to overcome the internal reisistance.
To increase current capacity, modern batteries have thin multiple plates which are connected in parallel within each cell, forming a kind of lead sandwich with a negative plate at each end and alternating positive plates within, all insulated from one another by separators. The separators are micro-porous, meaning charged ions can pass through readily, but they prevent physical contact that would constitute a short circuit, and also attempt to prevent the formation of the tiny alloy short circuits.
Steady improvements in separator materials have given us more powerful batteries without size increases.
Motorcycle batteries are normally rated by their amp/hour (AH) capacity, or cold-cranking amps. The AH figure is used in the advertising of car batteries as a months-of-life number, as in a 48-month or 60-month battery.
Figure that a12AH battery can produce one amp of current flow for 12 hours, or two amps for six hours, or 12 amps for one hour, etc. To calculate how long you'd have to leave your lights on to kill a fully charged battery, use this formula: Power in Watts (add the headlight and taillight) divided by Voltage (12 volts usually) equals current draw in Amps. For instance, a 60-watt high beam plus a 12 watt tail bulb equals 72 watts, divided by 12 volts equals a 6-amp draw. So your 12AH battery is stone dead in two hours. Of course, it will be too weak to start your bike in less than two hours.
The cold-cranking amp (CCA) rating indicates how many amps a battery can pass in 30 seconds at 0º Fahrenheit and is probably a big benchracing topic among snowmobile pilots. Actually, cold weather works a double hardship on your battery. Not only is your cold bike's engine oil thicker and harder to turn, but the battery loses power as well. A fully charged battery is only 65% as strong at 32º as it was at 80º, and at zero, it drops to only 40% capacity.
Batteries wait for their new owners in a state of hibernation called dry-charged, which means the manufacturer has dried the preformed positive and negative plates, assembled the battery and sealed it in a bag that doesn't contain oxygen. It can sleep like this for two or three years without a problem.
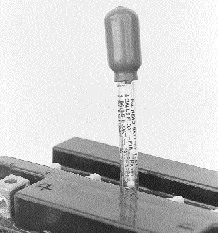 Simple floating ball hydrometer
indicates charge by the weight of the electrolyte. A fully charged batterys
electrolyte is 26% heavier than water, so the more of the graduated balls that
float, the higher the charge.
Simple floating ball hydrometer
indicates charge by the weight of the electrolyte. A fully charged batterys
electrolyte is 26% heavier than water, so the more of the graduated balls that
float, the higher the charge.
The biggest single harm that can be done to the life expectancy of a new battery usually occurs when it's sold. In the rush to get a new machine set up for sale or to get the customer out the door with his new replacement battery, many batteries are simply filled with electrolyte, installed and pressed into service within minutes of being unwrapped. Sound familiar? It's all wrong. What this procedure buys you is a battery that will never have more than 80% of its powerever! For this reason, many riders prefer to prep their new batteries themselves to make sure it's done correctly.
Here's the actual factory-recommended service procedure, and don't be too surprised if you've never heard it before.
First, unwrap the battery, unkink the vent tube and snip about a half-inch off the end, unless it's a maintenance-free type and doesn't have a vent tube. Next, fill it with electrolyte midway between the high and low level markings on the case. Next, let the battery sit for one or two hoursafter which the battery will still be at only 65% of full charge. Check the electrolyte level and add electrolyte if required. That's rightadd electrolyte. This is the only time in the life of your battery that you can ever add anything but water.
Nowsurpriseyou must still charge the battery. If it is a refillable lead-antimony type, it should be charged at one-third of its rated capacity in amp/hours for four to five hours to get it to full charge. Honda and Yuasa recommend that the maintenance-free type should be charged with a constant-current charger that can drive the charge with as much as 16.9 volts and closely monitored not to exceed full charge (another reason to buy a smart charger). Afterwards, recheck the level and add water if required. Finally, let the battery cool so the case contracts enough to fit into its typically tight little holder, run the new breather tube (if it has one) carefully through the original factory routing, being sure that it's well away from your chain and you're ready to go.
Although not so messy as your typical housepet discharge, we must always have our plastic and lead pal strong and full of energy, so it's still a problem. Because motorcycles are often simply recreational beasts and may have to wait a long time for our rare opportunities to give them some exercise, accessories that draw even small amounts of power like clocks or radio memories can sap the energy of even a big battery more quickly than we often imagine. You can measure the draw by disconnecting your positive battery terminal and connecting an ammeter in line.
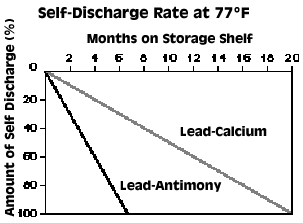
However, even a battery disconnected from its cables slowly self-discharges over time, typically at a rate of between .5% and 1% of total output per day for the refillable lead-antimony battery, or between .15% and .3% per day for the maintenance-free lead-calcium type. It's therefore prudent to charge your bike's battery every two to four weeks if the bike is not being used, more often if accessories are drawing power. The new smart chargers make the drudgery of this chore a thing of the past. Internal circuitry monitors the battery's condition and applies voltage only as necessary so your battery is always ready to go when you are. Stay tuned to MCN for a comprehensive battery charger tech feature and comparison test in the near future.
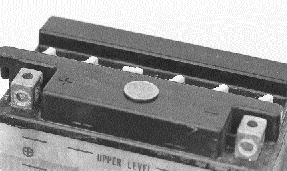 Try glueing a penny to the top of the
battery as a sacrificial anode to minimize corrosion of the
terminals.
Try glueing a penny to the top of the
battery as a sacrificial anode to minimize corrosion of the
terminals.
The discharge rate is also temperature-dependent. Hot weather greatly accelerates self-discharge (at 95º, it's twice as fast as at 77º, and 130º is considered lethal) and cold weather slows it, so it stands to reason that charged batteries are best stored in a cool (and dry) place. However, very cold weather can actualy turn electrolyte to jelly or even freeze it solid. And since a discharged battery contains a greater percentage of water, it freezes sooner than a fully charged one. Just a frosty 27º can be enough, but a fully charged battery will resist freezing down to a -75º. Also, don't attempt to jump-start a very cold battery without letting it thaw out first. You may have heard the story that a battery sitting on cement will discharge very quickly, and so it's best to store it on wood. This is no longer true, but once was when battery case materials were actually porous enough to enable discharge through damp cement.
Even if you're not experiencing any problems, routine battery inspection can be the ounce of prevention that saves many pounds of new lead cure. Periodic checks of the electrolyte level allow you add distilled water before the level drops below the tops of the plates. Should this happen, contact with the oxygen in the air will cause the sulfation, which is actually crystalline, to grow until it will bridge the plates and short them out permanently. And distilled water is an absolute necessity in a motorycle battery. The second worse thing you can do to hurt a battery is use tap water to top it up. Since you should already be keeping a jug of distilled water around for your bike's cooling system, you already have it for your battery, and should use it in your car's battery as well. You should also know that the electrolyte-level sensors fitted to many touring bikes should be replaced with the battery because the length, size and diameter vary from one manufacturer to another.
Simple corrosion of the battery terminals can drastically reduce the battery's ability to supply, receive or hold voltage. Plus, the gradual buildup of acid fumes, dirt and grime into an electrically-conductive film on the case between the terminals can greatly accelerate self-discharge. Baking soda mixed with warm water is the perfect tonic to cure these problems. Sponging this mixture over the area around the battery is a good idea too; rusting usually begins first where the paint on the seat base, frame and battery box are etched by the battery's fumes. The battery top should be kept clean, but be careful not to get baking soda inside the battery where it will neutralize your electrolyte. Carbonated soft drinks will also work to dissolve corrosion in a pinch, but you'll have to rinse away the sugar; or you'll soon be riding a high-speed ant farm.
It's wise to occasionally disconnect your battery cables and clean the connections even if they don't look bad. The surface you can't see is where the contact corrodes first. Brushing the soda solution on the corrosion produces a satisfying rapid white foaming as the crusty stuff is neutralized. Just apply until the foaming stops, dry the pieces and burnish the terminals with a wire brush or fine sandpaper to ensure an excellent connection. Try glueing a penny to the battery's top as a sacrificial anode to minimize corrosion.
Before bolting the connections back together, put a wipe of dielectric grease (available from auto parts stores) on the terminals to resist corrosion in the future. Vaseline also works if you can handle the bad jokes when friends find it in your toolbox.
A motorcycle battery should never receive the kind of high-rate booster charge intended for a car battery, and unfortunately that always seems to be the only type of charger a service station has on hand when your bike needs help.
 Inexpensive trickle charger with
pigtail connector allows easy battery charging. Do not leave these charging
longer than overnight without monitoring the battery.
Inexpensive trickle charger with
pigtail connector allows easy battery charging. Do not leave these charging
longer than overnight without monitoring the battery.
To learn the proper maximum charging rate for your battery, look at the alphanumeric code printed across the case of the battery and you will usually find its Amp/Hour rating. If it's not obvious, check your owners manual. The proper trickle-charging rate for a motorcycle battery is one-tenth of the A/H rating for as long as 10 hours, depending on how discharged it is. Charging faster than 2.02.5 amps causes overheating which can warp and even melt the battery case if ignored. High-rate charging also speeds up internal corrosion, and its visible sign is sediment buildup under the cells, which if it reaches high enough, will also permanently short out the battery. Too high a charging rate can also result in a battery that does not hold a charge because too-rapid transformation of the lead sulfate may actually trap sulfate under a surface coating of rejuvenated lead, producing a battery that can test okay but fails quickly. Thankfully, this last effect can be reversed with a very slow charge of no more than 1¼20 (yes, that's one-twentieth) of rated capacity for 25 to 30 hours.
Therefore, because we really don't want a powerful charger, a good battery charger for most purposes is the inexpensive low-output type. Typically selling for around $20, the so-called "trickle chargers" usually produce no more than 1.2 amps. They often incorporate a solid-state feedback circuit that will taper the charge down to even lower levels as the battery voltage comes back up, preventing overcharging. Most convenient are the quick-connect type that provide a pigtail connector that can be permanently attached to the battery. The trickle charger will also work on your car battery, but even more slowly, and produces the same battery-friendly results.
Both the taper-rate and trickle charger supply only a fixed voltage. However, the lead-antimony battery should be charged at 1415 volts, but the lead-calcium type needs l516 volts to reach full charge. What's the voltage of your charger? Does it match your battery type?
Constant current chargers like the Optimate or Battery Tender brands are called smart chargers because they can vary the charging voltage to keep current constant and charge a battery much more quickly. We'll cover their other advantages in a future article.
Carburetion, ignition and battery problems can often have similar syptoms, so it's important to test your battery properly before spending time and money on other fixes that may not produce results. First, visually inspect the side of the battery for proper electrolyte level, check its bottom for sediment buildup and take a look down the filler holes for evidence of extreme sulfation, which appears as a white crusty substance. Since the cells are connected in series, only one cell needs to short for the battery to be ruined.
Proper charging is the next step. The vent caps should be unscrewed and merely resting in their holes to allow positive venting without acid spattering from the bubbling electrolyte. During charging, a good battery's cells should all begin gassing (bubbling) together. If one cell begins rapid gassing before the others, it is likely to be a bad cell.
A voltmeter can be used to test the battery. Because charging creates what's called a surface charge at the top of the plates that can give a false indication of voltage strength, a charged battery should be installed in the bike and the headlight's high beam turned on for about three minutes to eliminate the surface charge. A voltmeter connected across the terminals of a conventional lead-antimony battery should show between 12.3 and 12.6 volts on a 12-volt battery and exactly half those numbers on a 6-volt battery. If the result is below these numbers, charging has been insufficient or you've got a bad battery (see chart). An even more reliable test can be made with a load on the charged battery. Turn on the ignition and headlight but don't start the engine, and check the voltage across the battery terminals. With this load the battery should show at least 11.2 volts on a 12-volt battery or 5.6 volts on a 6-volt battery. If the result is below 10 volts on a 12-volt or 4 volts on a 6-volt, you've got a bad cell and will have to replace the battery.
Less expensive than a voltmeter, and just as useful for indicating condition is a battery hydrometer. Hydrometers come in two styles: a fancy fully-graduated type (less than $20) that has great accuracy, or the simple floating ball type (about $7) that shows condition but without the numbers. Because the percentage of water in the electrolyte increases as the level of charge decreases, the hydrometer simply measures the weight of the electrolyte to determine the battery's state of charge. Plain water has a specific gravity of 1.000, and full-strength electrolyte is 1.260, or 26% heavier.
A simple chart converts the hydrometer reading into percent of charge. Some of the fancy graduated types even have a built-in thermometer to correct for electrolyte temperature deviation from the standard 77º. Although it's not a very significant factor, it works out to plus .003 for every 10º hotter than 77º, or minus .003 for every 10º cooler. Regardless of whether the battery is a 12-volt or a 6-volt, it should show at least 1.200 specific gravity or it needs charging. Always test every cell because individual cells that show a variation of more than .050 to one another indicate a bad cell that requires battery replacement. We can even determine cell voltage from the specific gravity reading, by adding .84 to the hydrometer number.
Unfortunately, most of the precise graduated hydrometers are made for car use, and their hoses are too big for the typical motorcycle battery's cell opening unless you make an adapter, and the volume of acid needed to fill them is more than most bike batteries contain above the cells.
The inexpensive floating-ball type is hardly bigger than an eyedropper, and made just for motorcycles. It contains five captive balls of progressive weight. If all five float on a lead-antimony battery, the battery is overcharged, four=full charge, three=75%, two=50%, one=25% and zero=dead. A variation of two balls between cells indicates a bad cell and will require battery replacement.
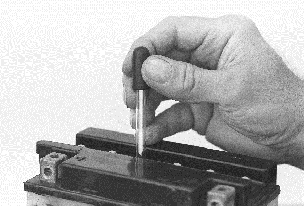 Drugstore eyedropper (two for $1)
makes a handy filler for adjusting electrolyte level with distilled
water.
Drugstore eyedropper (two for $1)
makes a handy filler for adjusting electrolyte level with distilled
water.
When testing a battery before charging, use the hydrometer before adding any water. Although these inexpensive testers are popular, you should know that if you keep them long enough, and use them often enough, the little balls can become waterlogged (or electrolyte-logged) and begin to indicate worse and worse battery conditions until you could end up throwing away a perfectly good battery. Rinsing these hydrometers in clear water after each use and air-drying them before popping them back in their little plastic sheaths should help, but periodically test them against a voltmeter reading or a new hydrometer to be sure.
Obviously, the lead-calcium maintenance-free battery does not allow access to the electrolyte for specific-gravity testing, so we must rely on voltage testing. However, we need another chart for these batteries because in addition to the change in the grid alloy, the electrolyte solution also contains more acid, producing greater voltage per cell, 2.2 instead of 2.1 volts. Therefore a maintenance-free battery should show at least 12.8 volts or it needs charging, and if it can't show more than 12.0 afterwards, it's bad.
When making battery connections, we want to prevent ignition of the dangerous gases that can be present inside the battery or around its vent holes or vent hose. During charging, the water inside the battery is broken into its hydrogen and oxygen elements. Recall that oxygen is the essential component for combustion. And if you've ever seen the famous newsreel of the great Hindenburg zeppelin going down in flames, remember that it was filled with lighter-than-air hydrogen, which is very explosive and which wants to rise out of the battery.
For this reason, you should not smoke or create any sparks near the battery, and please wear face protection. A face shield, even your full-face helmet, would be a good idea, no joke, or at least goggles for eye protection, and always keep your face away from the top of any battery you're working on unless you wouldn't mind having a complexion like a golf ball. If the gas does ignite, it will explode the battery, throwing acid in all directions. It really can happen. It really does happen. I know two people it's happened to. Be careful.
If your bike has a 12-volt battery, only jump it from another 12-volt battery. Be careful when making connections that the clamps do not touch each other or anything except battery terminals or the appropriate grounds. Do not let the two vehicles touch each other! Metallic contact could cause a common ground and possibly ignite the gasoline in the tanks! Turn off unnecessary lights and accessories, and if your bike is equipped with a radio, be sure to turn it off; voltage surges can erase or damage memory circuits. Connect the two batteries in parallel, positive-to-positive, negative-to-negative. Make the positive connections first, starting at the discharged battery, then connecting to the good battery, Next, connect the negative cable to the good battery's negative terminal, then the last clamp should not be connected to the negative terminal on the discharged battery, but attached to an engine bolt or fame ground on the dead bike away from the battery or its vent tube to minimize the chances of explosion. Don't use chromed or painted parts for the ground connection because they could be discolored. Once the bike has been started, the disconnections should be the exact reverse of the connection sequence. This exact same procedure also applies to cars.
Once the bike that required jump-starting is running, the owner will often leave the machine idling for an extended time in the mistaken belief that this will allow the battery to return to full charge. Unfortunately, aside from the potential engine damage that could result if the machine were air-cooled and thus overheating, or the choke were left on, so the over-rich fuel mixture washed the lubrication from the cylinder walls and created exceptional friction wear on the piston rings or cylinders, the alternator really doesn't do anything to charge the battery until engine rpm is considerably higher than idle, at what is called the break-even rpmthe point at which more current is being produced than what's necessary to run the lights and ignition system. Even routine use on trips of less than 15 or 20 miles is typically not enough to recharge the battery's losses from starting and self-discharge. Testing and thorough charging should always be your first response after needing a jump-start if you want to avoid a repeat performance.
We've seen that batteries are often neglected to death, just like goldfish, that charging procedures only appropriate for automobiles often get applied to the special case of motorcycle batteries, doing unnecessary damage, and that good safety procedure is not common knowledge. There's a lot to remember, so save this information, or clip the charts for your toolbox and share them with your friends.
Print out this page and stick it up in your garage near where you work on your bike. Refer to it every time you are checking, charging or replacing your battery. Remember, the better you care for it, the longer your battery will last, and the less likely you are to be left stranded.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Much of the technical data for this article was supplied courtesy of Yuasa-Exide, Inc., in its publication Battery Handbook and Technical Guide. For your own free copy of this handbook, contact Yuasa-Exide at P.O. Box 14145, Reading, PA 19612-4145; Tel: (610) 208-1916, Fax: (610) 208-1918. | Important Points to Remember
|
![]()
To
subscribe to Motorcycle
Consumer News Call 888-333-0354 —Home—Subscriptions—Staff—Back Issues— Copyright 1997 Motorcycle Consumer News |
![]()
Site and contents Copyright 1997 Motorcycle Consumer
News. All rights reserved. Reprinting in whole or in part expressly forbidden
except by written permission of the publisher or editor. Motorcycle Consumer
News (ISN 1073-9408) is published monthly by Aviation News Corporation, a
subsidiary of Fancy Publications, 3 Burroughs, Irvine, CA 92618. Corporate
headquarters located at P.O. box 57900, Los Angeles, CA 90057-0900; (213)
385-2222
Copyright 1997, Aviation News Corporation.